When we talk about “What is the pH”, we should remember that we are talking about science, not social science. This article will focus on the basic concept of pH, pH scale, pH values, and a few related numerical examples.
Table of Contents
What is the pH?
pH is denoted as “potential of Hydrogen”, which measures the acidity or basicity of an aqueous solution. pH is a measurement of the relative amount of free hydrogen and hydroxyl ions in an aqueous solution.
Origination of pH:
pH was first introduced by a Danish Chemist Søren Peter Lauritz Sørensen (S.P.L. Sørensen). He was a great Chemist and Scientist. He was born on 9th January 1868 and died on 12th February 1939. He introduced the pH scale and measurement system in 1909 but the modern pH system was revised to accommodate in terms of electrochemical cells in 1924.
Definition of pH:
Potential of Hydrogen (pH) can be defined as negative logarithmic of the hydrogen ion concentration.
In other words,
The logarithmic decimal of the reciprocal of the hydrogen ion (H+) concentration. A pH value below 7 indicates acidity, while a pH above 7 indicates alkalinity.
What is the pH scale?
The pH scale is a scale that measures and gives the nature of the solution. The scale measures the acidic or basic of the solution.
- The pH value of less than 7, indicates that the solution is acidic.
- A pH value greater than 7, indicates that the solution is basic or alkaline.
- The pH value equal to 7, indicates that the solution is neutral.

What is acidity?
Any solution that has a higher concentration of hydrogen ions (H+) compare to hydroxide ions (OH–), the solution will be acidic. A solution with a lower concentration of hydrogen ions (H+) that has higher pH is considered a basic solution.
What is the basicity?
If a solution has less concentration of hydrogen ions (H+) compared to hydroxide ions (OH–), the solution will be basic. In another word, if a solution has hydroxide ions (OH–) than hydrogen ions (H+), the solution will be basic or alkaline. A solution with a higher concentration of hydrogen ions (H+) that has lower pH is considered an acidic solution.
What is the importance of pH?
pH is a very important quantity that exhibits the chemical conditions of any solution. It plays an important role to see the acid and basic nature of a solution either solution belongs to an agricultural or chemical laboratory.
What are the applications of pH?
When we talk about the application of pH in a different area, the below names of the area come to mind.
-
Water treatment:
pH is an important parameter to know whether the water is drinkable or not. The pH range of water between 6.5 and 8.5 falls under the category of drinking water. By controlling pH, we can protect pipes, motors, and other accessories in the water treatment plant. Water with low pH can degrade pipes, causing toxic metals such as Cu and Pb to leach into the water supply. Whereas water with high pH gives an unpleasant taste.
-
Agriculture:
Knowing the complex system of soil leads helps in deciding the type of fertilizer to be used and the types of crops to sow. The pH of the soil gives the idea of different factors such as fungal growth, microbial activity, fungal growth, and root growth.
-
Swimming pool maintenance:
The pH value of water in the Swimming pool should be between the range of 7.2 to 7.8. The pH of water in a Swimming pool lower than 7.2 can irritate the eyes and nose.
-
Food Industry:
pH in the food industry is for the test of quality or monitoring of food quality.
-
In research:
Processes in the chemical and biological laboratory can be adjusted after knowing the pH of any solution. The process may be like sterilization, fermentation, enzyme hydrolysis, acidic hydrolysis, etc.

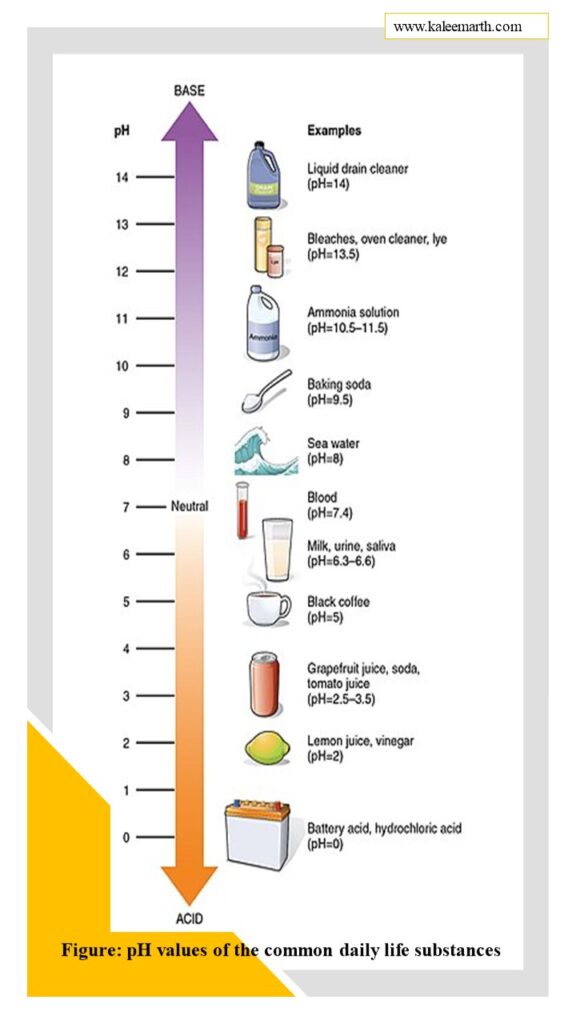
What are the pH values of the common daily life substances?
It is difficult to enlist the pH values of the common daily life substances. A pH of 7 is neutral, meaning neither acidic nor basic. This is the ideal range for growing healthy plants. When the pH drops below 7, the water becomes too acidic, making it toxic to plants. When the pH rises above 7, the water becomes overly basic, causing stress to the roots of the plant. But a few substances with pH values are given below:
How to calculate the pH?
pOH = – log [OH–]
pH = — log [H+]
[H+][OH-] = 1×10^-14
You can try these examples to test your knowledge of pH as below:
Example 1: What is the pH for a specific [H+] is given as = 1.5 x 10-5 M?
pH = -log10[H+]
pH = -log10(1.5 x 10-5)
pH = 4.233
Example 2: What will be the value of [H+] from a known pH = 6.5?
[H+] = 10-pH
[H+] = 10-6.5
[H+] = 3.16 x 10-7 M
Example 3: What is the pH of 0.1 M of NaOH solution?
0.1 M of NaOH is a strong base, that will produce 0.1 mol/L of OH ions in solution.
0.1 M = 0.1 mol/ lit
[ OH–] = 0.1 mol/lit of ions in solution
pOH = – log [OH–] =-log [ 0.1]
Can be written as:
pOH = −log [1×10−1]= − log [1] – log[10−1] = − log [1] – ( – 1× log 10) = 0 + 1 × 1 = 0 + 1 = 1
pOH =1
we know that
pH + pOH = 14
pH + 1 = 14
pH = 13
Example 4: What is the pH of 1 M of NaOH solution?
1 M = 1 mol/ lit
[ OH–] = 1 mol/lit of ions in solution
pOH = – log [OH–] =-log [1]
pOH =0
we know that
pH + pOH = 14
pH + 0 = 14
pH = 14
Sources:
- Alberty, Robert; Silbey, Robert (1996). Physical Chemistry (second ed.). John Wiley & Sons, Inc. p. 244. ISBN978-0-471-10428-5
- Helmenstine, Anne Marie, Ph.D. “pH Definition and Equation in Chemistry.” ThoughtCo, Jul. 29, 2021, thoughtco.com/definition-of-ph-in-chemistry-604605.


1 thought on “What is the pH, pH value, and pH scale, and how does it work? 1. Basic concept with solution”