Welcome to our blog post on the Introduction of Mass Transfer Operation, one of the fundamental topics in Chemical Engineering. Mass transfer is the movement of a component in a substance from one location to another, typically from a high concentration to a low concentration. It plays a crucial role in various industrial processes such as distillation, absorption, adsorption, and more. In this article, we will provide a basic approach to understanding mass transfer in chemical engineering and how it can be applied in real-world scenarios. So, let’s dive in and explore the world of mass transfer!
Table of Contents
What is the Mass Transfer?
Mass transfer is the process by which mass is transferred from one place to another, typically through a fluid medium. It involves the movement of one or more components of a mixture from one phase or location to another, such as from a gas phase to a liquid phase, or from one liquid phase to another. The driving force for mass transfer is typically a concentration gradient, which results from a difference in the concentration of the components in the different phases or locations. Mass transfer plays a critical role in many industrial processes, such as distillation, absorption, extraction, and drying.
The basic concept of Mass Transfer
The basic concept of mass transfer involves the movement of one or more components of a mixture from one phase or location to another. The driving force for mass transfer is typically a concentration gradient, which results from a difference in the concentration of the components in the different phases or locations. The concentration gradient drives the movement of the components from a region of high concentration to a region of low concentration.
Mechanism of Mass Transfer
Mass transfer occurs through different mechanisms, including diffusion, convection, and reaction. Diffusion is the process by which the components move due to the concentration gradient. Convection involves the movement of the components due to the bulk motion of the fluid. The reaction involves the conversion of one component into another through a chemical reaction.
Diffusion
Diffusion is the primary mechanism of mass transfer in most industrial processes. It occurs when the concentration gradient is created by the difference in concentration between two regions. The below figure shows a concept to understand the Diffusion process.
The rate of diffusion is proportional to the concentration gradient, the diffusion coefficient, and the area through which the mass is transferred.

Convection
Convection involves the movement of the components due to the bulk motion of the fluid. Convection can be either forced or natural. Forced convection occurs when the fluid is moved by an external force, such as a pump or a fan. Natural convection occurs when the fluid moves due to density differences caused by temperature or concentration gradients.
Reaction
The reaction involves the conversion of one component into another through a chemical reaction. The rate of reaction is typically proportional to the concentration of the reactants, the reaction rate constant, and the surface area of the reaction. The reaction can be either homogeneous or heterogeneous, depending on whether the reactants are in the same phase or different phases.
Mass Transfer Coefficient
The rate of mass transfer is typically determined by the mass transfer coefficient, which depends on factors such as the properties of the components, the properties of the medium, and the geometry of the system. The mass transfer coefficient represents the effectiveness of the transfer of mass from one phase or location to another.
The mass transfer coefficient can be determined experimentally or theoretically. Experimental determination of the mass transfer coefficient involves measuring the rate of mass transfer under controlled conditions and using this data to calculate the coefficient. Theoretical determination of the mass transfer coefficient involves using mathematical models to predict the rate of mass transfer based on the properties of the components, the properties of the medium, and the geometry of the system.
Industrial Applications of Mass Transfer
Mass transfer plays a critical role in many industrial processes, such as distillation, absorption, extraction, and drying.
Distillation is a process in which a liquid mixture is separated into its components by boiling the mixture and condensing the vapors. The separation is based on the difference in boiling points of the components. Mass transfer plays a critical role in distillation, as it is the driving force for the separation of the components.
Absorption is a process in which a gas mixture is contacted with a liquid to remove one or more components from the gas. Mass transfer plays a critical role in absorption, as it is the driving force for the transfer of the components from the gas phase to the liquid phase.
Extraction is a process in which a component is separated from a mixture by contacting the mixture with a solvent that preferentially dissolves the component. Mass transfer plays a critical role in extraction, as it is the driving force for the transfer of the component from the mixture to the solvent.
Formulas in Mass Transfer
There are several important formulas in the mass transfer that are used to calculate various parameters related to the transfer of mass. Here are some of the most used formulas:
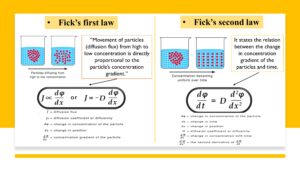
Fick’s First Law
This formula describes the rate of diffusion of a species in a fluid medium. It can be written as:
J = -D(dC/dx)
Where J is the flux of the species, D is the diffusion coefficient, C is the concentration of the species, and x is the distance.
Fick’s Second Law
This formula describes the change in concentration of a species over time due to diffusion. It can be written as:
dC/dt = D(d^2C/dx^2)
Where t is time, x is distance, and D is the diffusion coefficient.
Both of Fick’s laws can be understood from the below figure.
Sherwood Number
This formula describes the mass transfer coefficient for diffusion. It can be written as:
Sh = KdL/D
Where Sh is the Sherwood number, Kd is the mass transfer coefficient for diffusion, L is a characteristic length scale, and D is the diffusion coefficient.
Nusselt Number
This formula describes the mass transfer coefficient for convection. It can be written as:
Nu = hL/k
Where Nu is the Nusselt number, h is the heat transfer coefficient, L is a characteristic length scale, and k is the thermal conductivity.
Schmidt Number
This formula describes the ratio of momentum diffusivity to mass diffusivity. It can be written as:
Sc = ν/D
Where Sc is the Schmidt number, ν is the kinematic viscosity, and D is the diffusion coefficient.
Reynolds Number
This formula describes the ratio of inertial forces to viscous forces in a fluid. It can be written as:
Re = ρVL/μ
Where Re is the Reynolds number, ρ is the density of the fluid, V is the velocity of the fluid, L is a characteristic length scale, and μ is the dynamic viscosity.
These formulas are essential for understanding and designing mass transfer operations in various industries, such as chemical engineering, biotechnology, and environmental science.
Important Question and Answer from Mass Transfer
Important Question and answer from mass transfer operation in chemical engineering, which might be useful for competitive exams such as GATE and interviews.
Question: What is the difference between diffusion and convection?
Answer: Diffusion is the movement of molecules from a region of high concentration to a region of low concentration due to random molecular motion, while convection is the transfer of mass due to the bulk motion of the fluid.
Question: What is molecular diffusivity?
Answer: Molecular diffusivity is a measure of the rate at which molecules diffuse through a fluid. It is expressed in units of length squared per unit of time.
Question: What is Fick’s first law of diffusion?
Answer: Fick’s first law of diffusion states that the flux of a diffusing species is proportional to the concentration gradient of the species.
Question: What is Fick’s second law of diffusion?
Answer: Fick’s second law of diffusion describes how the concentration of a diffusing species changes with time.
Question: What is the Sherwood number?
Answer: The Sherwood number is a dimensionless quantity that relates the mass transfer coefficient to the molecular diffusivity.
Question: What is the Schmidt number?
Answer: The Schmidt number is a dimensionless quantity that relates the momentum diffusivity to the molecular diffusivity.
Question: What is the Lewis number?
Answer: The Lewis number is a dimensionless quantity that relates the thermal diffusivity to the molecular diffusivity.
Question: What is the mass transfer coefficient?
Answer: The mass transfer coefficient is a measure of the rate at which mass is transferred across a boundary between two phases.
Question: What is the film theory of mass transfer?
Answer: The film theory of mass transfer assumes that mass transfer occurs through a stagnant film that is in contact with a moving fluid.
Question: What is the penetration theory of mass transfer?
Answer: The penetration theory of mass transfer assumes that mass transfer occurs due to the diffusion of species through a boundary layer.
Question: What is the difference between the mass transfer coefficient and the overall mass transfer coefficient?
Answer: The mass transfer coefficient is a measure of the rate at which mass is transferred across a boundary between two phases, while the overall mass transfer coefficient takes into account the resistances to mass transfer in both the liquid and gas phases.
Question: What is the difference between steady-state and unsteady-state mass transfer?
Answer: In steady-state mass transfer, the rate of mass transfer remains constant over time, while in unsteady-state mass transfer, the rate of mass transfer changes with time.
Question: is the boundary layer thickness?
Answer: The boundary layer thickness is the distance from a surface at which the velocity of a fluid is equal to 99% of the free-stream velocity.
Question: What is the mass transfer rate?
Answer: The mass transfer rate is the amount of mass that is transferred across a boundary between two phases per unit of time.
Question: What is the driving force for mass transfer?
Answer: The driving force for mass transfer is the concentration gradient between the two phases.
Question: What is the diffusivity of a gas?
Answer: The diffusivity of a gas is a measure of the rate at which the gas diffuses through a fluid.
Question: What is the Stefan-Maxwell equation?
Answer: The Stefan-Maxwell equation relates the diffusivities of different species in a mixture.
Question: What is the Knudsen number?
Answer: The Knudsen number is a dimensionless quantity that relates the mean free path of a gas molecule to a characteristic length scale.
Question: What is the boundary layer?
Answer: The boundary layer is the region adjacent to a surface where the velocity of a fluid is affected by the presence of the surface.
Question: What is the difference between mass transfer and heat transfer?
Answer: Heat Transfer is concerned with the transfer of heat, whereas Mass Transfer is concerned with the transfer of mass.
Question: What is the mass transfer?
Answer: The mass transfer is the movement of one or more components of a mixture from one phase to another phase.
Question: What are the driving forces of mass transfer?
Answer: The driving forces of mass transfer are concentration, temperature, and pressure gradients.
Question: What is the mass transfer coefficient?
Answer: The mass transfer coefficient is the proportionality constant between the mass transfer rate and the driving force.
Question: What is Fick’s law?
Answer: Fick’s law states that the mass flux is proportional to the concentration gradient.
Question: What is the equation for the mass transfer rate?
Answer: The mass transfer rate equation is given by N = kA(C1-C2), where N is the mass transfer rate, k is the mass transfer coefficient, A is the area of the interface, and C1 and C2 are the concentrations of the species at the interface.
Question: What is the Sherwood number?
Answer: The Sherwood number is the dimensionless ratio of the mass transfer coefficient to the molecular diffusivity.
Question: What is the Schmidt number?
Answer: The Schmidt number is the dimensionless ratio of the momentum diffusivity to the molecular diffusivity.
Question: What is the Lewis number?
Answer: The Lewis number is the dimensionless ratio of the thermal diffusivity to the molecular diffusivity.
Question: What is the mass transfer rate of a gas?
Answer: The mass transfer rate of a gas is given by
N = kA(P1-P2)
where N is the mass transfer rate, k is the mass transfer coefficient, A is the area of the interface, and P1 and P2 are the partial pressures of the species at the interface.
Question: What is the mass transfer rate of a liquid?
Answer: The mass transfer rate of a liquid is given by.
N = kA(C1-C2)
where N is the mass transfer rate, k is the mass transfer coefficient, A is the area of the interface, and C1 and C2 are the concentrations of the species at the interface.
Question: What is the boundary layer thickness?
Answer: The boundary layer thickness is the distance from the interface to the point where the concentration or velocity of the species reaches 99% of the bulk concentration or velocity.
Question: What is the interfacial area?
Answer: The interfacial area is the total area of the interface between two phases.
Question: What is the mass transfer coefficient for laminar flow?
Answer: The mass transfer coefficient for laminar flow is given by k = 2D/δ, where D is the diffusion coefficient and δ is the boundary layer thickness.
Question: What is the mass transfer coefficient for turbulent flow?
Answer: The mass transfer coefficient for turbulent flow is given by
k = 0.664 (D/δ)^1/3 (Re)^1/2,
k = 0.664 (D/δ)1/3 (Re)1/2
where D is the diffusion coefficient, δ is the boundary layer thickness, and Re is the Reynolds number.
Question: What is the Reynolds number?
Answer: The Reynolds number is a dimensionless quantity that relates the inertial forces to the viscous forces in a fluid flow.
Question: What is the Schmidt number for a gas?
Answer: The Schmidt number for gas is given by
Sc = μ/ρD
where μ is the dynamic viscosity, ρ is the density, and D is the diffusion coefficient.
Question: What is the Schmidt number for a liquid?
Answer: The Schmidt number for a liquid is given by.
Sc = μ/ρD
where μ is the dynamic viscosity, ρ is the density, and D is the diffusion coefficient.
Question: What are the three mechanisms of mass transfer?
Question: What is the classification of mass transfer operation?
Answer: Diffusion, absorption, leaching, extraction, adsorption, and drying are all parts of mass transfer.
Question: What is the unit of mass transfer?
Answer: Units of mass transfer coefficient are measured in moles per unit of volume.
Question: What is mass transfer also known as?
—————-*************——————-


1 thought on “Introduction of Mass Transfer Operation || Basic Approach || Chemical Engineering || 2023”