Petroleum refineries and petrochemicals play a significant role in our daily lives. From the fuel we use in our cars to the plastics that make up the containers for our food and drinks, these industries are essential to modern society. Chemical engineers are at the forefront of this field, working to develop new methods and technologies for refining petroleum and producing petrochemicals. In this blog post, we’ll provide an introduction to these industries and explore their basics and applications in the chemical engineering field.
Table of Contents
Introduction
Petroleum refineries and petrochemicals are integral components of the global energy industry, playing a vital role in converting crude oil and natural gas into a diverse range of products that power our modern society. Petroleum refineries are responsible for processing crude oil into refined products such as gasoline, diesel, jet fuel, and various chemicals, while petrochemical plants convert hydrocarbons into essential building blocks for plastics, fibers, pharmaceuticals, and more. This article provides an in-depth exploration of petroleum refineries and petrochemicals, delving into their processes, products, significance, and impact on our daily lives.
Petroleum Refineries
-
Overview
Petroleum refineries are industrial facilities that receive crude oil as feedstock and transform it into various refined products. They are complex plants comprising multiple units and processes, each serving a specific purpose in the refining process. The primary goal of a refinery is to separate crude oil into its constituent hydrocarbon components and convert them into marketable products.
-
Crude Oil Distillation
Crude oil distillation is the foundational process in a petroleum refinery, where crude oil is separated into different fractions based on their boiling points. The distillation tower or column plays a crucial role in this process by utilizing the varying boiling points of hydrocarbons to achieve separation.
-
Conversion Processes
Refineries employ various conversion processes to upgrade the quality and yield of products. These include catalytic cracking, hydrocracking, catalytic reforming, and alkylation, among others. Each process has its specific purpose, such as breaking down heavy hydrocarbons, producing high-octane gasoline, or converting low-value components into valuable petrochemical feedstocks.
-
Treatment Processes
Treatment processes, including hydrotreating and desulfurization, are utilized to remove impurities and enhance the quality of refined products. These processes play a crucial role in meeting environmental regulations and producing cleaner fuels.
-
Product Distribution
Refined products from the refinery are distributed through various channels, including pipelines, tanker trucks, and railcars, to reach end-users such as gas stations, airports, and industries. Petroleum refineries produce a wide range of refined products that serve as fuels for transportation, heating, and industrial processes. These products include gasoline, diesel, jet fuel, liquefied petroleum gas (LPG), heating oil, asphalt, and various other specialty products. Gasoline and diesel, in particular, are the most widely used fuels for automobiles, trucks, ships, and airplanes, providing the energy needed for transportation across the globe.
Petrochemicals
-
Introduction
Petrochemicals are chemical compounds derived from petroleum or natural gas. They serve as the building blocks for a vast range of products used in diverse industries. Petrochemical plants employ chemical processes to convert hydrocarbon feedstocks into different chemicals with specific properties.
-
Steam Cracking
Steam cracking is a fundamental process in petrochemicals, where large hydrocarbon molecules are broken down into smaller, more valuable molecules such as ethylene, propylene, and butadiene. These molecules serve as essential feedstocks for the production of plastics, synthetic fibers, and elastomers.
-
Polymerization
Polymerization is a process wherein monomers are chemically linked together to form polymers. This process is critical in the production of plastics, as different polymerization techniques yield various types of plastics with distinct properties.
-
Alkylation and Aromatization
Alkylation involves combining smaller hydrocarbon molecules, such as propylene and isobutane, to produce high-octane gasoline components. Aromatization, on the other hand, converts naphtha into aromatic compounds like benzene, toluene, and xylene, which serve as feedstocks for the production of solvents, plastics, and synthetic fibers.
-
Petrochemical Derivatives
Petrochemical derivatives include a wide range of chemicals used in various industries. These derivatives encompass products such as ethylene glycol (used in antifreeze and polyester fibers), polyethylene (used in packaging materials), polypropylene (used in automotive parts and textiles), and styrene (used in the production of polystyrene and rubber). These derivatives find applications in sectors such as automotive, construction, packaging, textiles, pharmaceuticals, agriculture, and more.
Processes in petroleum refining
Certainly! Here are some of the processes commonly used in petroleum refining, along with examples:
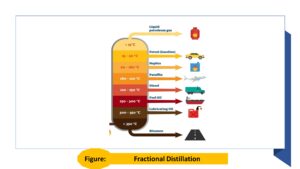
Distillation: The process of separating crude oil into different fractions based on their boiling points. Examples of fractions obtained through distillation include gasoline, diesel, kerosene, and fuel oil. The fractional distillation can be understood from figure.
Catalytic Cracking: This process breaks down heavy hydrocarbon molecules into lighter ones by using a catalyst. For example, heavy gas oil can be converted into gasoline, diesel, and other lighter products.
Hydrocracking: It is a more severe form of catalytic cracking that involves the use of hydrogen to break down heavy hydrocarbons. It is used to produce high-quality gasoline, diesel, and jet fuel.
Isomerization: This process converts straight-chain hydrocarbons into branched-chain isomers, which have higher octane ratings. For example, n-pentane can be isomerized to isopentane.
Alkylation: It involves combining an olefin, such as propylene or butylene, with an isoparaffin in the presence of an acid catalyst to produce high-octane gasoline components called alkylates.
Reforming: The process of converting low-octane naphtha into high-octane gasoline components through the use of heat and a catalyst, such as platinum or rhenium.
Desulfurization: This process removes sulfur compounds from petroleum products to meet environmental regulations. It is typically done through hydrodesulfurization, where the product is treated with hydrogen over a catalyst.
Polymerization: It involves the combination of small hydrocarbon molecules to form larger molecules known as polymers. For example, ethylene can be polymerized to produce polyethylene, which is used in plastic production.
Isomerization: This process converts straight-chain hydrocarbons into branched-chain isomers, which have higher octane ratings. For example, n-pentane can be isomerized to isopentane.
Hydrogenation: It is a process that adds hydrogen to unsaturated hydrocarbons to produce saturated hydrocarbons. For example, hydrogenation can convert vegetable oils into solid fats, such as margarine.
These are just a few examples of the processes used in petroleum refining. The selection and combination of these processes depend on the desired product slate and the properties of the crude oil being processed.
Formulas in Petroleum and refining
Certainly! Here are some formulas used in petroleum refining and petrochemicals, along with examples:
Octane Number
The octane number is a measure of a fuel’s resistance to knocking or detonation in an internal combustion engine. It indicates the fuel’s ability to resist premature combustion, which can cause engine damage and reduced efficiency. The higher the octane number, the greater the fuel’s resistance to knocking. The octane number is typically determined using two methods: Research Octane Number (RON) and Motor Octane Number (MON). The formulas for RON and MON are as follows:
- Research Octane Number (RON):
RON = (A + B + C + D) / 2
- Motor Octane Number (MON):
MON = (E + F + G + H) / 2
In these formulas, A, B, C, D, E, F, G, and H are the percentages of specific hydrocarbons in the fuel mixture. These hydrocarbons have different chemical structures and combustion characteristics, and their contributions are combined to calculate the octane number.
For example, let’s consider a fuel sample with the following hydrocarbon percentages:
A = 8, B = 12, C = 22, D = 55, E = 2, F = 0.5, G = 1, H = 1.5
Using the RON formula: RON = (8 + 12 + 22 + 55) / 2 = 97.5
Using the MON formula: MON = (2 + 0.5 + 1 + 1.5) / 2 = 2.5
In this case, the octane number of the fuel would be RON 97.5 and MON 2.5.
Cetane Number
The cetane number (CN) is a measure of the ignition quality of diesel fuel. It is determined through standardized testing methods, such as the ASTM D613 test. The cetane number is calculated based on the results of the test, which compares the ignition delay of the test fuel with that of reference fuels of known cetane numbers. The formula to calculate the cetane number is as follows:
CN = 0.8 × (Tc + 46.3)
In this formula, Tc represents the ignition delay time in degrees Celsius, measured during the cetane number test. The ignition delay time is the time between fuel injection and the start of combustion. For example, let’s consider a cetane number test where the ignition delay time is measured as 3.5 degrees Celsius. Using the formula:
CN = 0.8 × (3.5 + 46.3) = 0.8 × 49.8 = 39.84
In this case, the cetane number of diesel fuel would be approximately 39.84.
Gross Refining Margin (GRM)
The gross margin for refining GRM is the difference between the price of crude oil as the raw material (input) and the total value of petroleum products leaving an oil refinery (output). Per-barrel calculations are used to determine the margins.
GRM = (Total Value of Petroleum Products Produced – Total Cost of Crude Oil Processed) / Total Quantity of Crude Oil Processed
Example: If the total value of petroleum products produced is $10,000, the total cost of crude oil processed is $8,000, and the total quantity of crude oil processed is 1,000 barrels, then the GRM would be ($10,000 – $8,000) / 1,000 = $2 per barrel.
Yield Percentage
Yield percentage is a measure of the efficiency or effectiveness of a chemical process or reaction in producing a desired product. It represents the percentage of the desired product obtained from a given number of raw materials or feedstock.
Yield Percentage = (Product Yield / Feedstock Quantity) × 100
Example: If the product yield is 500 tons and the feedstock quantity is 1,000 tons, then the yield percentage would be (500 / 1,000) * 100 = 50%.
Density Calculation
Density is a fundamental property of a substance that relates its mass to its volume. It is commonly calculated using the formula:
Density = Mass / Volume
In this formula, the mass refers to the mass of the substance being considered, and the volume refers to the volume occupied by that mass.
Example: If the mass of a liquid is 1,000 kg and the volume is 1 m^3, then the density would be 1,000 kg / 1 m3 = 1,000 kg/m3.
API Gravity
API Gravity, also known as the American Petroleum Institute gravity, is a measure of the density of petroleum liquids relative to the density of water. It is commonly used in the petroleum industry to classify and compare different types of crude oil and petroleum products.
The API Gravity is determined based on the specific gravity of the petroleum liquid, which is the ratio of the density of the liquid to the density of water at a specified temperature. The formula to calculate API Gravity is:
API Gravity = (141.5 / Specific Gravity at 60°F) – 131.5
In this formula, the specific gravity is dimensionless and represents the density of the liquid relative to the density of water at the same temperature.
Significance and Impact
The petroleum refining industry and petrochemical sector play a crucial role in modern society, with significant significance and impact. Here are some key points highlighting their importance:
Energy Supply
Petroleum refineries and petrochemicals provide the energy supply necessary for transportation, heating, and industrial processes. Refined products, particularly gasoline and diesel, fuel vehicles, and machinery, enable the movement of goods and people across the globe.
Economic Growth
The petroleum refining and petrochemical industries contribute significantly to economic growth and employment. They create jobs in refining and petrochemical plants, supporting industries, and associated sectors such as logistics, engineering, and construction. Moreover, these industries generate revenue through exports, trade, and investments.
Product Diversity
Petroleum refineries and petrochemicals enable the production of a wide range of products, meeting the diverse needs of society. From fuels that power vehicles to plastics that revolutionize packaging, construction materials, and consumer goods, these industries provide essential materials for various sectors, driving innovation and technological advancements.
Environmental Considerations
While petroleum refining and petrochemicals have been associated with environmental concerns, significant efforts have been made to address these issues. Refineries have implemented technologies to reduce emissions, improve energy efficiency, and produce cleaner fuels. Petrochemical plants are exploring sustainable feedstocks and investing in recycling and circular economy initiatives to minimize waste and environmental impact.
- Emissions and Pollution Control:
Petroleum refineries and petrochemical plants are conscious of their environmental impact and have implemented measures to reduce emissions and pollution. They employ technologies such as catalytic converters, desulfurization units, and waste treatment systems to minimize the release of harmful pollutants into the atmosphere and water bodies. Continuous research and development in cleaner technologies aim to further improve environmental performance.
- Sustainable Practices:
The industry is increasingly adopting sustainable practices to minimize its carbon footprint. This includes the utilization of renewable energy sources, implementation of energy-efficient processes, and recycling and reuse of waste materials. By promoting circular economy principles, the industry strives to reduce waste generation, conserve resources, and minimize environmental impact.
- Carbon Capture and Utilization:
In response to the global focus on reducing greenhouse gas emissions, petroleum refineries and petrochemical plants are exploring carbon capture and utilization (CCU) technologies. These technologies aim to capture carbon dioxide emissions and convert them into valuable products such as chemicals, fuels, and construction materials. CCU has the potential to contribute to both emissions reduction and the development of a circular carbon economy.
Future Perspectives: Trends and Challenges
Sustainability and Renewable Feedstocks:
The petroleum refining and petrochemical industries are increasingly focusing on sustainability and renewable feedstocks. The industry is exploring the use of renewable feedstocks, such as biomass and waste materials, to produce biofuels and bio-based chemicals. This shift towards renewable resources aligns with the growing demand for sustainable and environmentally friendly alternatives.
Advanced Process Technologies:
Advancements in process technologies, such as process optimization, digitalization, artificial intelligence process intensification, nanotechnology, and biotechnology, are revolutionizing the petroleum refining and petrochemical sectors. These technologies offer more efficient processes, improved product yields, and reduced energy consumption, leading to cost savings and environmental benefits also enable better control over processes, predictive maintenance, and resource optimization.
Circular Economy Approach:
The adoption of a circular economy approach aims to maximize resource efficiency by recycling and reusing waste materials. This approach minimizes waste generation and reduces reliance on virgin feedstocks, thereby contributing to sustainability and resource conservation.
Important questions and answers
Certainly! Here are some short questions and answers related to petroleum refining, that might be useful for interviews and written tests.
Question: What is petroleum refining?
Answer: Petroleum refining is the process of converting crude oil into various useful products such as gasoline, diesel, jet fuel, and petrochemical feedstocks.
Question: What is a refinery?
Answer: A refinery is an industrial facility where crude oil is processed and refined to produce different petroleum products.
Question: What are the main objectives of petroleum refining?
Answer: The main objectives of petroleum refining are to separate crude oil into its various components, remove impurities, and transform the components into valuable products.
Question: What are the primary processes in petroleum refining?
Answer: The primary processes in petroleum refining include distillation, cracking, reforming, isomerization, alkylation, and hydrotreating.
Question: What is distillation in petroleum refining?
Answer: Distillation is a process that separates crude oil into different fractions based on their boiling points.
Question: What is cracking in petroleum refining?
Answer: Cracking is a process that breaks down heavy hydrocarbon molecules into lighter, more valuable products such as gasoline and diesel.
Question: What is reforming in petroleum refining?
Answer: Reforming is a process that converts low-octane naphtha into high-octane gasoline.
Question: What is hydrotreating in petroleum refining?
Answer: Hydrotreating is a process that removes sulfur, nitrogen, and other impurities from petroleum products using hydrogen.
Question: What are petrochemicals?
Answer: Petrochemicals are chemical compounds derived from petroleum that are used as building blocks in the production of various products, including plastics, rubber, fibers, and chemicals.
Question: What is the importance of petroleum refining?
Answer: Petroleum refining is essential for meeting the global demand for transportation fuels and providing raw materials for the petrochemical industry.
Question: What are the environmental challenges in petroleum refining?
Answer: Environmental challenges in petroleum refining include managing air emissions, treating wastewater, and minimizing the environmental impact of by-products such as sulfur and carbon dioxide.
Question: What is the role of technology in petroleum refining?
Answer: Technology plays a crucial role in improving efficiency, optimizing processes, and developing cleaner and more sustainable refining practices.
Question: What are the major products obtained from petroleum refining?
Answer: The major products obtained from petroleum refining include gasoline, diesel, jet fuel, heating oil, lubricants, asphalt, and petrochemical feedstocks.
Question: How are petroleum products distributed and transported?
Answer: Petroleum products are distributed and transported through a network of pipelines, tankers, barges, and trucks to reach end-users such as fuel stations, airports, and industrial facilities.
Question: What are the challenges facing the petroleum refining industry?
Answer: Challenges facing the petroleum refining industry include fluctuating crude oil prices, changing demand patterns, regulatory compliance, and the need for continuous technological advancements.
Question: What is the role of catalysts in petroleum refining?
Answer: Catalysts are substances that facilitate chemical reactions in petroleum refining processes, such as cracking and reforming, by lowering the activation energy required for the reactions to occur. They help improve process efficiency and increase the yield of desired products.
Question: What is the significance of sulfur removal in petroleum refining?
Answer: Sulfur removal is crucial in petroleum refining to meet environmental regulations and reduce the emission of sulfur compounds, which contribute to air pollution and the formation of acid rain. Processes like hydrotreating are employed to reduce the sulfur content in petroleum products.
Question: What are the challenges in producing cleaner-burning fuels?
Answer: Producing cleaner-burning fuels involves reducing the levels of pollutants like sulfur, nitrogen compounds, and particulate matter. This requires the use of advanced refining techniques and the development of catalysts that can effectively remove these impurities.
Question: How does the refining process impact the quality of petroleum products?
Answer: The refining process can enhance the quality of petroleum products by adjusting their composition, octane or cetane numbers, and physical properties to meet specific performance requirements. For example, refining can improve the octane rating of gasoline for better engine performance.
Question: What is the relationship between petroleum refining and the petrochemical industry?
Answer: Petrochemicals are derived from petroleum refining by further processing and transforming certain fractions or byproducts into chemicals used in various industries. Petrochemical plants often coexist with petroleum refineries to maximize the utilization of resources.
Question: How does the choice of crude oil impact the refining process?
Answer: Different types of crude oil have varying compositions and properties, such as sulfur content, viscosity, and density. The choice of crude oil affects the complexity of the refining process and determines the yield and quality of the resulting products.
Question: What is the role of process optimization in petroleum refining?
Answer: Process optimization involves maximizing the efficiency and profitability of petroleum refining operations. It entails adjusting operating parameters, optimizing catalyst usage, and implementing advanced control systems to achieve optimal process performance.
These questions and answers cover various aspects of petroleum refining and its significance in the industry. The field continues to evolve as technologies advance and environmental considerations become more critical.
Conclusion
Petroleum refineries and petrochemicals are integral parts of the global energy industry, providing essential fuels and chemical products that drive our modern society. Refineries transform crude oil into a wide range of refined products, while petrochemical plants convert hydrocarbons into valuable building blocks for numerous industries. These industries play a vital role in economic development, job creation, and technological advancements. Moreover, they are adapting to meet environmental challenges and exploring sustainable practices to ensure a greener and more sustainable future. As we move forward, the petroleum refining and petrochemical sectors will continue to evolve, embracing innovation and driving positive change in the energy landscape.
———-*********———–
Read also
- Chemical Engineering || Introduction || Carrier 2023
- Introduction to Heat Transfer || Chemical Engineering
- Mass Transfer || Chemical Engineering
- Introduction to Chemical Reaction Engineering
- Basics of Chemical Engineering Thermodynamics
- Fluid Flow Operation aka Fluid Mechanics
- Chemical Process Industries
————–*******—————

