The success of hydrogen-based stuff such as hydrogen-powered cars, and fuel cells on the market depends on the creation of clean, sustainable, and affordable hydrogen production and generation systems. This article will be covering and explore 7 cutting-edge technological possibilities for hydrogen production.
Table of Contents
What is hydrogen?
Hydrogen is the cleanest fuel we have ever seen. Hydrogen is produced naturally from water and is abundant in nature. However, we need to find ways to produce it efficiently and safely. Hydrogen is a chemical element that occurs naturally in small amounts in many substances. Click here to know more about hydrogen.
What are the 3 basic technologies for hydrogen production?
The three major categories of different processes of hydrogen production as below:
- Thermal process
- Electrolysis process
- Photolytic process
Each processing method has its advantages and disadvantages, each of these methods with its pros and cons is already discussed (click here). Let’s take a look at 7 cutting-edge technological possibilities for hydrogen production to drown from the above three major categories. Read more about 3 major categories of different processes of hydrogen production (Click here).
Seven cutting-edge technological possibilities for hydrogen production
The seven important production technologies drawn from three major categories are briefly discussed category-wise. Under thermal process four major technologies for hydrogen production are summarized as follows:
1. Distributed Natural Gas Reforming (DNGR):
The most practical method for introducing hydrogen into the automobile markets shortly is natural gas reformation at gas stations. Natural gas reforming is a sophisticated production method that expands on the natural gas pipeline delivery infrastructure already in place. Today, huge central plants using natural gas reforming create 95% of the hydrogen generated in the US. This is a crucial technological route for producing hydrogen soon. Distributed Natural Gas Reforming (DNGR) is also called steam-methane reforming (SMR).
- DNGR is a well-developed manufacturing method in which hydrogen is made from a methane source, such as natural gas, using high-temperature steam (700°C–1,000°C).
- In the process of steam-methane reforming, the methane reacts with steam under pressures ranging from 3 to 25 in presence of a catalyst to produce hydrogen. Carbon monoxide and a negligibly small quantity of carbon dioxide are also produced with hydrogen.
- SMR is an endothermic process, heat must be added to the process for it to continue.
- Then, using a catalyst, the carbon monoxide and steam are reacted to create carbon dioxide and additional hydrogen in a process known as the “water-gas shift reaction.” See below the chemical reaction.
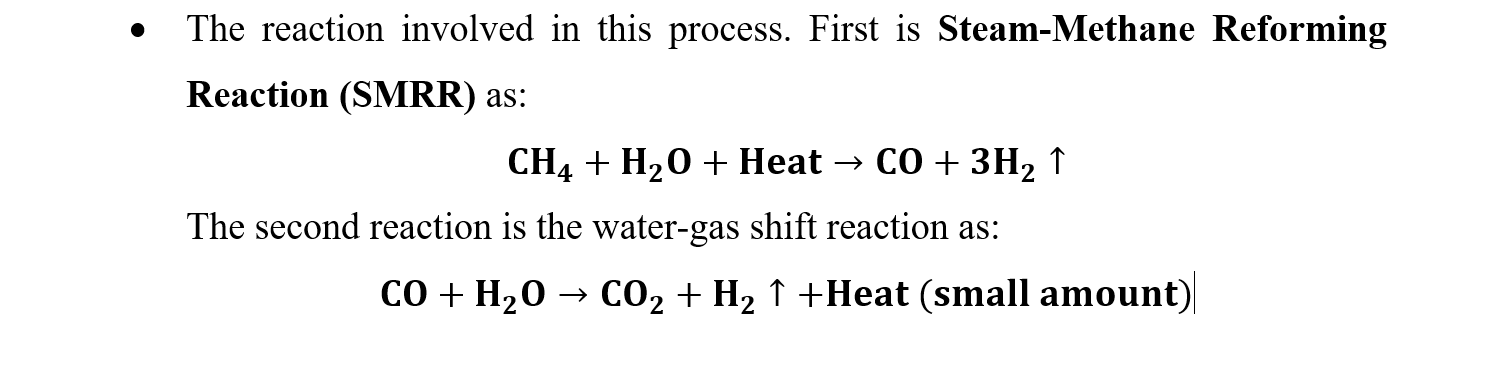
- The reaction involved in this process. First is Steam-Methane Reforming Reaction (SMRR) as:
- Carbon dioxide and other impurities are eliminated from the gas stream in the final step of the process, known as “pressure-swing adsorption,” leaving pure hydrogen.
- Hydrogen can also be made from other fuels, such as ethanol, propane, or even gasoline, using the same process called steam reforming.
2. Bio-Derived Liquids Reforming (BDLR):
The steps involved in the BDLR process to produce hydrogen are quite similar to those involved in Distributed Natural Gas Reforming (DNGR) process.
- In the presence of a catalyst, the liquid fuel reacts with steam at high temperatures to create a reformated gas that is primarily made of hydrogen, carbon monoxide, and a small amount of carbon dioxide.
- In the presence of a catalyst, the liquid fuel (ethanol, biofuels, etc.) reacts with steam at high temperatures to create a reformated gas that is primarily made of hydrogen, carbon monoxide, and a small amount of carbon dioxide.
- The carbon monoxide (made in the first step) is reacted with high-temperature steam in the “water-gas shift reaction,” which results in the production of additional hydrogen and carbon dioxide.

- Similarly, Carbon dioxide and other impurities are eliminated from the gas stream in the final step of the process, known as “pressure-swing adsorption,” leaving basically for pure hydrogen.
3. Coal and Biomass Gasification (CBG):
- As we know, almost any carbon-based fuel can be disassembled into its component chemicals using the gasification process.
- High temperatures (>700°C) are used during the gasification process to transform organic or fossil-based carbonaceous materials without combustion.
- It can be achieved using high pressures and temperatures, modern gasifier systems subject coal or biomass to hot steam and controlled amounts of air or oxygen. A simple example of a reaction for glucose, the actual biomass has a highly variable composition, cellulose is one of the major components of biomass.

- Finally, the hydrogen in this gas stream can be extracted using adsorbers or certain membranes.
4. Thermochemical Production (TCP):
- TCP is a heat-driven chemical reaction to split the water for hydrogen production.
- High-temperature heat (500° to 2,000°C) is used in TCP water splitting systems to drive a series of chemical reactions that yield hydrogen.
- The necessary required high temperatures can be achieved either by using concentrated sunlight or waste heat from advanced nuclear reactors.
- Using water and either sunlight or nuclear energy, high-temperature thermochemical water-splitting cycles may create hydrogen with almost no emissions of greenhouse gases.
Under the electrolytic process one major technology for hydrogen production is discussed below:
5. Water Electrolysis:
Using electricity, the process of electrolysis separates water into hydrogen and oxygen.
- The use of electricity to split water is a promising method of producing hydrogen.
- In this process, an electric current is passed through the water to split it into hydrogen and oxygen.
- This reaction takes place in a unit called an electrolyzer.
- Electrolyzers can range in size dependent on the production capacity.
- Electrolyzers are made up of an anode and a cathode separated by an electrolyte just like fuel cells.
- Although it is less efficient than a direct chemical path, this process produces almost no pollution or toxic byproducts if the electric current is generated using renewable energy.
- Different electrolyzers work in various ways, primarily due to the various electrolyte materials involved and the ionic species it conducts.
- The different electrolyzers are polymer electrolyte membranes (PEM), solid oxide electrolyzers, and alkaline electrolyzers.
The two main technologies for producing hydrogen are summarised as follows under the Photolytic process:
6. Photoelectrochemical (PEC) hydrogen production:
This process involves the directly split of water into hydrogen and oxygen using solar power and an appropriate semiconductor.
- Using sunlight and a special class of semiconductor materials, hydrogen can be produced directly from the water.
- The sun’s energy is used to directly dissociate water molecules into hydrogen and oxygen.
- PEC semiconductor materials are like those used in photovoltaic solar electricity generation. However, for PEC applications, the semiconductor is immersed in a water-based electrolyte, which is energized by sunlight.
- The PEC method requires a substance (photocatalyst) that should be extremely tough and effective to produce photoelectrochemical hydrogen.
- Currently, to overcome the obstacles posed by materials, scientists are investigating a variety of strategies, such as the use of metal doping, nanomaterial coatings, and other hybrid materials.
- Although research into PEC water splitting is still in its infancy, it has the potential to produce sustainable hydrogen with low environmental effects.
7. Biological Hydrogen Production (BHP):
Microorganisms and sunlight are used in the photobiological hydrogen production process to convert water into hydrogen.
- BHP is also called Photobiological Water Splitting.
- In BHP the sunlight is being used by bacteria to divide water into hydrogen and oxygen ions.
- The bacteria such as green microalgae or cyanobacteria.
- Hydrogen ions can be combined directly or indirectly and released as hydrogen gas.
- These microorganisms consume water and generate hydrogen as a byproduct of their normal metabolic processes, as like same as plants make oxygen through the photosynthesis process.
- BHP is a longer-term technological approach that is still in the early phases of study and has the potential to produce sustainable hydrogen in the future without severe environmental impacts.
Read more articles:
The potential role of artificial neural networks in chemical engineering



3 thoughts on “Hydrogen production roadmap: Exploring 7 cutting-edge technological possibilities”